AAMI Consensus Reports Guide ‘Bubble Helmet’ Development as COVID-19 response
August 5, 2020
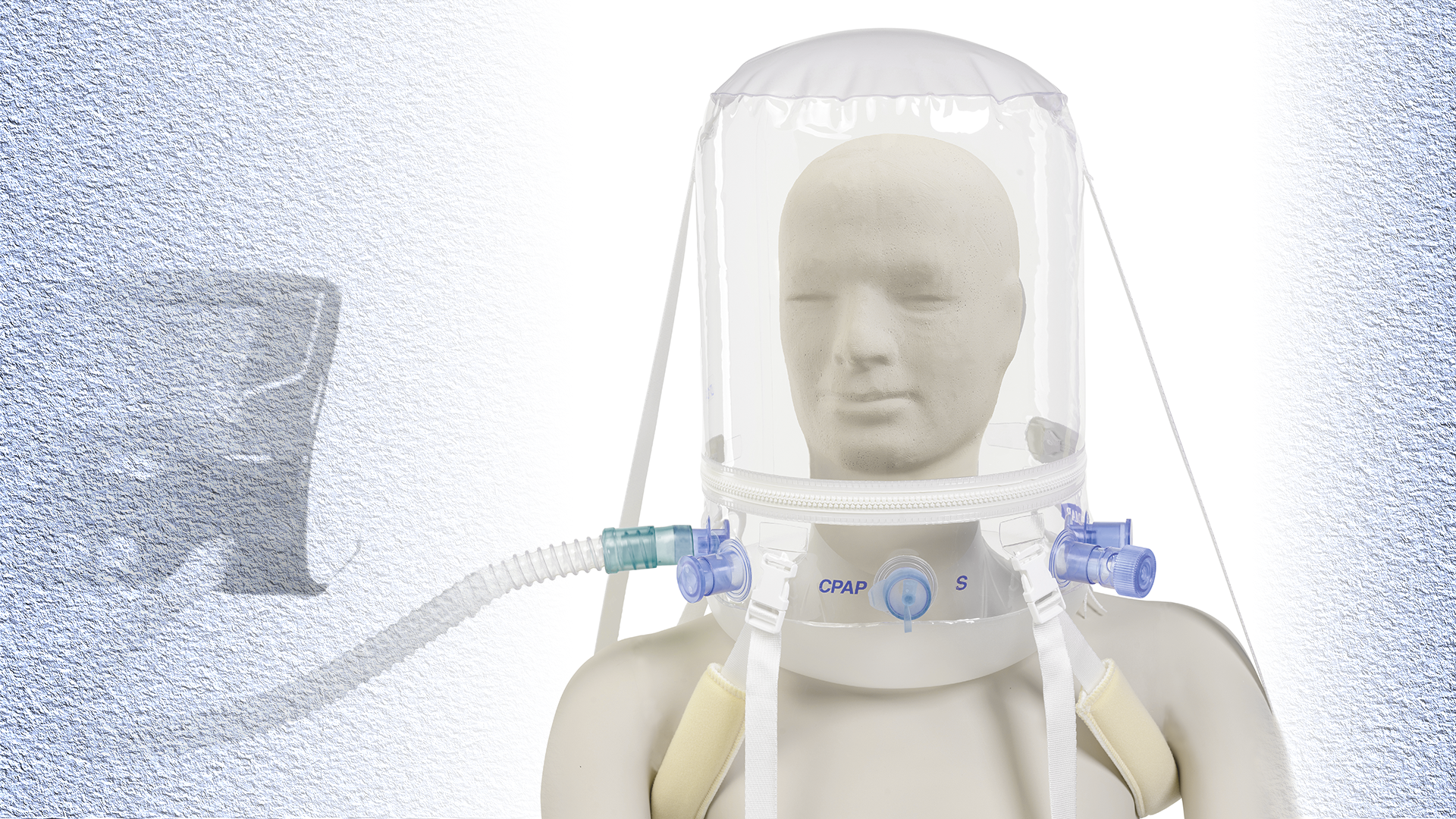
An example of a ventilatory assistance helmet already in production for emergency use in Italy. Non Invasive Ventilation Helmet. DIMAR S.r.l. – Medolla – Italy
For Immediate Release
Contact: Brian Stallard, bstallard@aami.org, (703) 647-2771
As the COVID-19 pandemic continues to afflict the world, innovative medical device designs are receiving emergency use authorizations from the US Food and Drug Administration (FDA), including for production of ventilatory assistance helmets (VAHs). Now, the Association for the Advancement of Medical Instrumentation (AAMI) has released two consensus reports (CRs) to guide manufacturers in making safe and reliable VAHs.
Bob Kopotic, senior manager of clinical affairs for critical care at Edwards Lifesciences, is quick to point out that the bubble-like VAH looks like it belongs in a 1950s sci-fi comic.
“It’s like a ‘space suit’ in Flash Gordon, right? You see someone with this clear, pressurized bubble around their head and they’re otherwise just wearing their normal clothes,” he said.
Kopotic, who co-authored the new CRs, is a registered nurse and respiratory therapist and serves on the AAMI COVID-19 Response Team. He explained that the novelty of the VAH design may lead to a high demand for the devices as the pandemic continues.
“Some of these helmets allow a patient to walk around. They can see their caregiver or the TV, and right now, since many hospitals are not letting relatives into the room, a patient can comfortably video chat with their loved ones and be recognized,” he said.
The VAH cannot replace a traditional mechanical ventilator. What makes a SARS-CoV-2 infection lethal is how it restricts a patient’s ability to breathe. By maintaining a pressurized flow of oxygen, VAH technologies are designed to reduce the work lungs do to keep breathing. However, they will never entirely do the breathing for a patient. Instead, a VAH can delay the need for invasive mechanical ventilation while drug therapy takes effect.
“Once somebody's on a mechanical ventilator, the number of staff having to care for them goes up. That’s because the patient then requires intubation, deep sedation, fluid therapy, and other needs,” Kopotic said. “But if a patient can live through acute respiratory failure and never graduate to needing full mechanical ventilation, that’s a lot of resources saved for the next patient.”
Still, a VAH comes with cautions. The latest AAMI CR notes that VAHs can use upwards of 80 liters of oxygen per minute to operate. This flow can quickly exhaust gas reserves in hospitals unprepared to support such high-flow systems. VAH manufacturers also need to warn caretakers of the dangers of an oxygen enriched environment such as fire risks from certain electronics and even skin lotions.
It’s also important to note that VAH technology is not unique to COVID-19 use, as they are common in hyperbaric therapy. However, during this global pandemic, several AAMI COVID-19 Response Team members have been asked to consult on emergency VAH directives and assist the FDA with issuing emergency use authorization. As a group, they determined that a pair of AAMI CRs would be a boon to industry and medical professionals alike.
“We are not dictating medical practice,” Kopotic said. “We just want to make sure that a good clinician is not going to hurt somebody with a device that has been inappropriately designed.”
The consensus reports AAMI CR508:2020, Emergency use Ventilatory Assistance Helmet (VAH) Design Guidance and AAMI CR509:2020, End user Disclosures for Emergency use Ventilatory Assistance Helmet (VAH) can be found on the AAMI COVID-19 Emergency Guidance web page alongside seven other consensus reports developed in response to COVID-19.
AAMI (www.aami.org) is a nonprofit organization founded in 1967. It is a diverse community of more than 10,000 healthcare technology professionals united by one important mission—supporting the healthcare community in the development, management, and use of safe and effective health technology. AAMI is the primary source of consensus standards, both national and international, for the medical device industry, as well as practical information, support, and guidance for health technology and sterilization professionals.
